What Are the 3 Parts of a Nucleotide and Nucleotide Examples
Nucleic acid like DNA and RNA are made up of nucleotide. A nucleotide is defined as an organic molecule found in living organisms. It is also the building block of RNA and DNA that makes life possible. DNA is able to store genetic information in the cells of living organisms. A nucleotide is found in various diverse molecules such as adenosine triphosphate (ATP), cyclic AMP (cAMP) and coenzymes such as NADP and NAD that play a role in metabolism. Apart from storing of genetic information, metabolism nucleotides assist in enzyme reactions, energy transport, and cell signaling.
Three Parts of Nucleotide
Below is a detailed elaboration of 3 parts of a nucleotide.
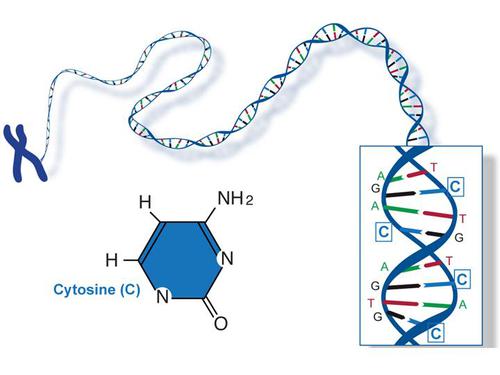
1. Nitrogenous base
A nucleotide molecule is made up of nitrogen, and because of the presence of nitrogen atom electrons, it is considered a base. The Nucleotide nitrogenous base is grouped into two categories, purines, and pyrimidines. Guanine and adenine are purines while Uracil, cytosine, and thymine are pyrimidines. Cytosine (C) and Adenine (A) are found in both DNA and RNA. However, a difference between DNA and RNA comes in when Thymine (T) found in DNA is replaced by Uracil (U) in RNA.
The main role of the nitrogenous base in the nucleotide structures is to carry information. The nitrogenous base is arranged in a way which ensures molecules can maximumly interact with each other while being exposed to different body functions at group levels.
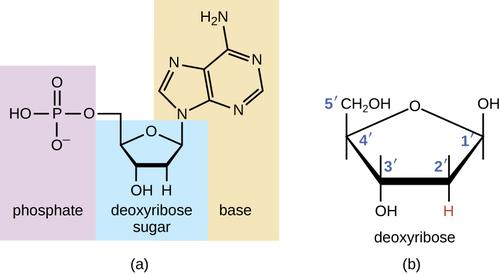
2. Sugar
The composition of sugar differs in DNA and RNA. For DNA, it contains 2'-deoxyribose of sugar, which is made of deoxyribose (5-carbon sugar). The ribose sugar found in RNA is made up of 5-carbon sugar as well. The sugar found in DNA and RNA gives them their full names; deoxyribonucleic acid and ribonucleic acid respectively. The chronological arrangement of carbons assists in monitoring where these groups get attached.
When sugar is exposed to oxygen, it joins the phosphate group of the next molecule that forms the sugar-phosphate backbone. Sugar-phosphate structure backbone aids in rigidity since covalent bonds are stronger than hydrogen bond. The sugar molecule acts as a decision maker on whether a nucleotide will form part of RNA molecule or a DNA molecule.
3. Phosphate group
PO43- is among the 3 parts of a nucleotide. It stands for one single phosphate group where the central atom is the phosphorus atom. An atom of oxygen is usually attached to the phosphorus atom and 5-carbon within the sugar as well. A phosphate group called adenosine triphosphate (ATP) is formed after phosphate groups are joined together to form a structure of P-O-P-O-P-O.
ATP assists living organisms in transmitting energy between reactants and storage of energy. ATP is composed of 3 phosphate groups that stores energy in their bonds. The bond formed is known as phosphodiester. It is formed when pentose sugars and phosphate groups bond together. The phosphodiester bond makes a structure similar to a ladder on the sides of the DNA because when hydrogen bonds with nitrogen bases, it becomes weak. The phosphate bond is also responsible for creating new genetic molecules that can be passed from one generation to the next.
Various Nucleotide Examples
Within the 3 parts of a nucleotide, there are five types of nucleotide bases which are: Adenine(A), Cytosine (C), Guanine (G), Thymine (T) and Uracil (U). When they are combined with sugar, they form nucleotide adenosine, cytidine, guanosine, thymidine, and uridine.
1. Adenine
Adenine belongs to the purine group of nitrogenous bases and has a double ring structure. Adenine bonds with thymine in DNA while in RNA it bonds with uracil. Energy is stored between phosphate groups since adenosine triphosphate (ATP) attaches the three phosphate groups using nucleotide adenine as a base. The energy stored in ATP and sugar-phosphate make their backbone very strong. That is the same bond types which get rid of energy by transferring it to molecules and other reactions through combination with special enzymes.
2. Guanine
Guanine is a nucleotide of the purine group found in nitrogenous bases and has a double ring structure like adenine. Its symbol is G. C5H5N5O represents the chemical formula of purine guanine. Guanine only bonds with cytosine in both DNA and RNA. Three hydrogen bonds make it possible for guanine to bond with cytosine. The presence of the three hydrogen bonds makes the cytosine-guanine bond stronger than a thymine-adenine bond because only two hydrogen bonds are involved in the latter.
3. Cytosine
Cytosine belongs to the pyrimidines group of nitrogenous bases which is one among the three parts of a nucleotide. It has a single ring structure. Its symbol is C. C4H5N3O represents the chemical formula of pyrimidine cytosine. Cytosine only bonds with guanine in both RNA and DNA because they make the strongest pairs. Cytosine can easily convert to Uracil, and when the mutation is not repaired fast enough, it will trace in the DNA. To change ADP to ATP, an enzyme cofactor known as Cytidine triphosphate (CTP) takes center stage.
4. Thymine
Thymine is one of the pyrimidine found in the nitrogenous bases and has one ring just like cytosine. Its symbol is T. C5H6N2O2 represents the chemical formula of pyrimidine thymine, and it's only found in DNA. Thymine- adenine is a weak pair because of the two hydrogen bonds formed.
5. Uracil
Uracil is another example of pyrimidine. Its symbol is U and is known as a weak acid. Its chemical formula is C4H4N2O2. Uracil is short-lived which is why most creatures do not use it in DNA but in RNA because RNA is a short-lived molecule as well. Uracil also goes everywhere thymine would go.
In conclusion, nucleotides are the building blocks of life. They perform different metabolic functions to make life possible. If asked 'what are the three parts of nucleotides?' you can now tell they are nitrogenous base, sugar and phosphate group. They are part and parcel of human life and very vital. Studying nucleotides does help in understanding human genetic composition as well as different physiological processes.
YOU MAY LIKE
-
Disadvantages and Advantages to Keep Animals in Zoos
-
5 Steps to Draw Lewis Structures
-
Do You Know Why People Don’t Read Books?
-
Most Interesting Challenges to Do With Your Friends
-
3 Types of Elements on the Periodic Table and Their Properties
-
Tips on How to Write a “Thank You” E-mail
-
11 Amazing Chemical Reactions in Everyday Life!
-
The Significance of Knowing Your Strengths and Weaknesses
-
Different Truth or Dare Questions for Adults
-
DNA VS RNA: A Comparative Study
